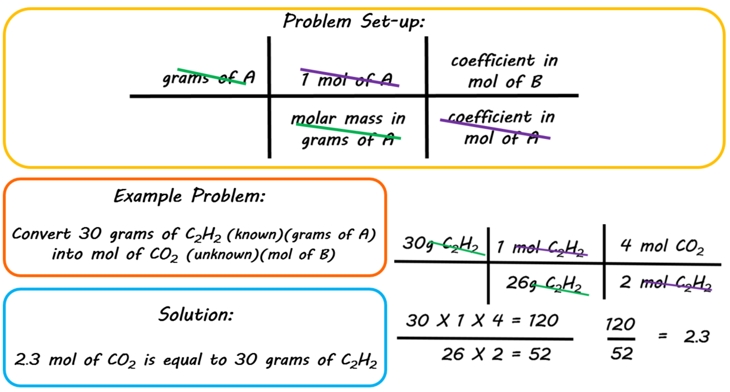
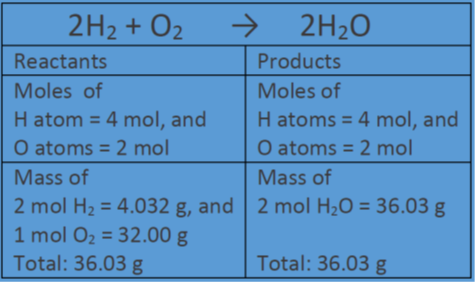
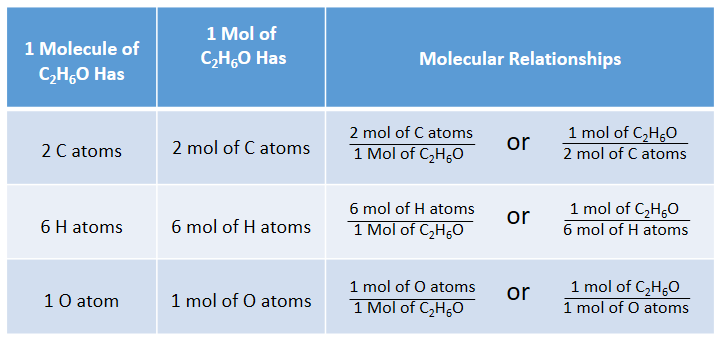
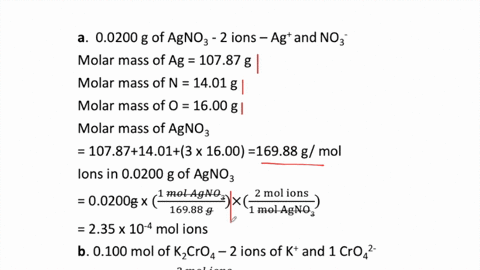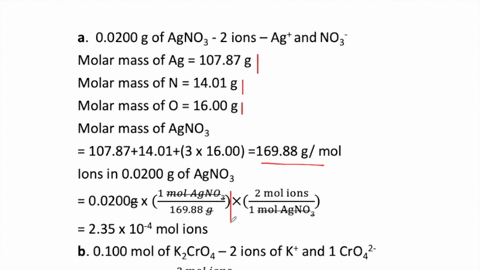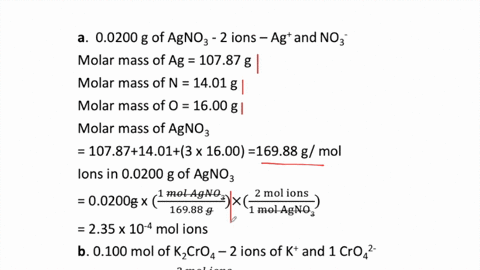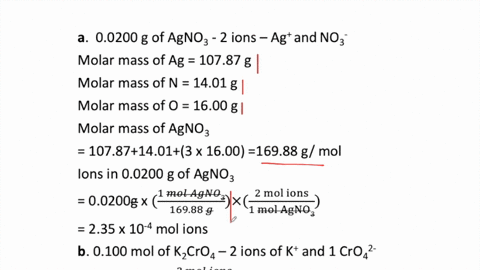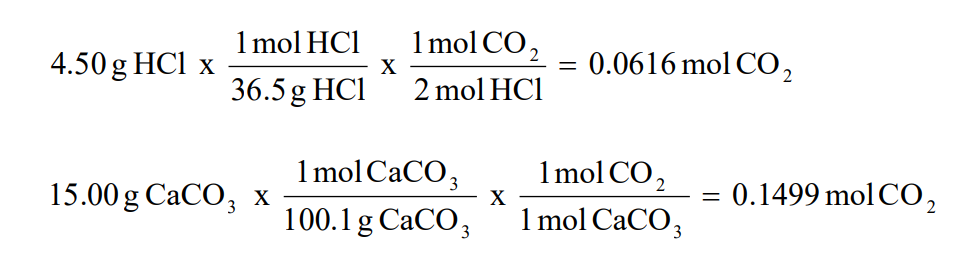THE “MOLE” A Mole (mol) is a unit of measurement used for counting very small things (atoms, molecules, etc.) A mole of something is similar to a dozen. - ppt download

For a chemical reaction 2X + Y → Z, the rate of appearance of Z is 0.05 mol L^-1 min^-1 . The rate of disappearance of X will be:

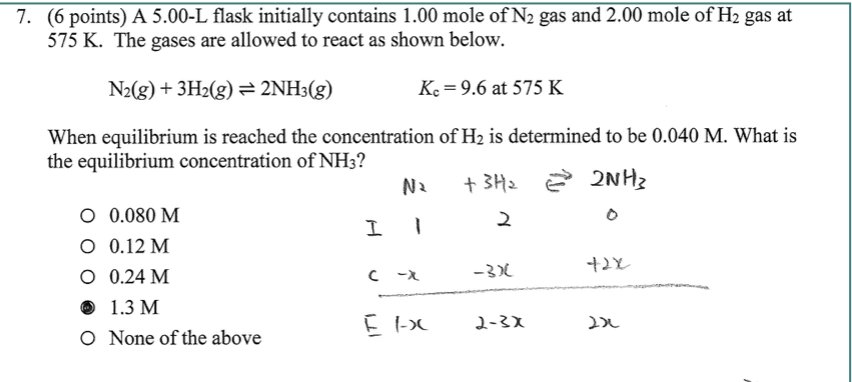
1 mole of N2 and 2 moles of H2 are allowed to react in a 1 dm^3 vessel. At equilibrium 0.8 mole of NH3 is formed. The concentration of H2 in the vessel is